Chromatography
Allowable Adjustments to HPLC Methods
Chromservis HPLC columns corresponding to USP and Eur. Pharmacopoeia methods and the extent to which the various parameters of a chromatographic test may be adjusted without fundamentally modifying the pharmacopoeial analytical procedures are listed in this technical note. Changes other than those indicated require revalidation of the procedure.
Sample loading in Flash chromatography
Liquid or solid, with small or large sample volumes: because your challenges are just as varied as your injections, we offer multiple possibilities for optimisation, to guarantee you the best results day after day.
Dry Load & Accessories
So far only available in a disposable plastic version, the Dry Loads are now also available in a stainless steel version. These are reusable and also have the advantage of greater resistance to pressure.
With the stainless steel Dry Loads, you can carry out solid injections on a preparative column and in flash column applications where the maximum pressure resistance of a plastic Dry Load would be insufficient.
Injection loops
Thanks to our range covering volumes from 100 μl to 50 mL, there is always an injection loop suited to your needs.
 Autosampler
Autosampler
Automate your injections with our autosampler and increase your performance tenfold. The puriFlash® AS-1 allows the injection of samples from 500 µl to 500 mL, with an automated cleaning of the transfer tubes between each injection. The injection is managed by a sample queue on InterSoft software. The autosampler xan be equiped with 6-way or 10-way electric valve.
One rack/slot offers capacities fo the test tubes/bottles up to 250 mL and custom racks.
 Injection Pump
Injection Pump
When the quantity of product to be injected becomes important and when having to use a syringe and multiply the injections for a single purification becomes constraining, the injection pump is the ideal tool. You just have to prime the pump with the product and then launch the method. It’s as easy as that!
HPLC Phases
ASTRA - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| C18-HE | 2, 3, 5, 10 | 100 | 330 | 17 | 2-9 |
| C18-AQ | 2. 3, 5 | 100 | 330 | 13 | 2-9 |
| C18-BDS | 3, 5 | 140 | 170 | 11 | 2-8 |
| C8-HE | 5 | 100 | 330 | 11 | 2-9 |
| C8-BDS | 3, 5 | 140 | 170 | 6 | 2-8 |
| Phenyl-Hexyl-HE | 3, 5 | 100 | 330 | 11 | 2-7.5 |
| DM | 3, 5 | 100 | 205 | 12 | 2-9 |
| Diol | 3, 5 | 100 | 330 | 5 | 2-7.5 |
ARION - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Plus C18 | 1.7, 2.2, 3, 5, 10, 15 | 100 | 420 | 18 | 1.5-10 |
| Polar C18 | 2.2, 3, 5, 10, 15 | 120 | 325 | 16 | 1.5-7.0 |
| C8 | 3, 5 | 120 | 325 | 11 | 2.0-7.0 |
| Phenyl-butyl | 2.2, 3, 5 | 100 | 300 | 12 | 1.5-7.5 |
| NH2 | 2.2, 3, 5 | 120 | 325 | 5 | 2.0-6.5 |
| CN | 3, 5, 10 | 120 | 325 | 8 | 2.0-7.0 |
| HILIC Plus | 2.2, 3, 5 | 120 | 420 | - | 1.5-7.0 |
| Si | 2.2, 3, 5, 10 | 100 | 420 | - | 1.5-7.0 |
| SAX | 5 | 120 | 325 | - | 1.0-7.5 |
| SCX | 5 | 120 | 325 | - | 1.0-7.5 |
More information is available in Column care guide aswell.
CHROMSHELL - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| CHROMSHELL® C18 Plus | 2.6 | 85 | 130 | 9 | 1.5-7.5 |
| CHROMSHELL® C18-XB | 2.6 | 85 | 130 | 8 | 1.5-8.0 |
| CHROMSHELL® C18 Polar | 2.6 | 85 | 130 | 6.5 | 1.5-7.0 |
KINETEX - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Effective Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Kinetex XB-C18 | 5, 2.6 | 100 | 200 | 10 | 1.5-8.5* |
| Kinetex C18 | 5, 2.6 | 100 | 200 | 12 | 1.5-8.5* |
| Kinetex C8 | 2.6 | 100 | 200 | 8 | 1.5-8.5* |
| Kinetex PFP | 5, 2.6 | 100 | 200 | 9 | 1.5-8.5* |
| Kinetex HILIC | 2.6 | 100 | 200 | 0 | 2.0-7.5 |
| Kinetex Phenyl-Hexyl | 5, 2.6 | 100 | 200 | 11 | 1.5-8.5* |
* Columns are pH stable from 1.5 to 10 under isocratic conditions. Columns are pH stable from 1.5 to 8.5 under gradient conditions.
Kinetex 2.6µm columns with ID 2.1mm are pressure stable up to 1000 bar, otherwise up to 600 bar.
Kinetec chore-shell colums can be replace by new ChromShell colums. Just try it.
LUNA - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Luna Phenyl-Hexyl | 3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L11 |
| Luna Silica (2) | 3,5,10,15 | 100 | 400 | - | - | L3 |
| Luna C5 | 5,10 | 100 | 440 | 12.5 | 1.5-10.0 | - |
| Luna C8 | 5,10 | 100 | 440 | 14.75 | 1.5-10.0 | L7 |
| Luna C8 (2) | 3,5,10,15 | 100 | 400 | 13.5 | 1.5-10.0 | L7 |
| Luna C18 | 5,10 | 100 | 440 | 19 | 1.5-10.0 | L1 |
| Luna C18 (2) | 2.5,3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L1 |
| Luna CN | 3,5,10 | 100 | 400 | 7.0 | 1.5-10.0 | L10 |
| Luna NH2 | 3,5,10 | 100 | 400 | 9.5 | 1.5-11.0 | L8 |
| Luna SCX | 5,10 | 100 | 400 | 0.55% Sulfur Load | 2.0-7.0 | L9 |
| Luna HILIC | 3,5 | 200 | 200 | - | 1.5-8.0 | - |
| Luna PFP(2) | 3 5 | 100 | 400 | 5.7 | 1.5-8.0 | L43 |
GEMINI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Gemini C18 | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
| Gemini C6-Phenyl | 3,5 | 110 | 375 | 12 | 1.0-12.0 | L11 |
| Gemini NX | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
SYNERGI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Synergi Max-RP | 2.5 | 100 | 400 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 2.5 | 100 | 400 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 2.5 | 100 | 440 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 2.5 | 100 | 440 | 12 | 1.5-10.0 | L1 |
| Synergi Max-RP | 4,10 | 80 | 475 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 4,10 | 80 | 475 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 4,10 | 80 | 475 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 4,10 | 80 | 475 | 12 | 1.5-10.0 | L1 |
ONYX - PHENOMENEX
| Packing Material | Macropore Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Onyx Silica | 2 | 130 | 300 | 0 | 2.0-7.5 | - |
| Onyx C8 | 2 | 130 | 300 | 11 | 2.0-7.5 | - |
| Onyx C18 | 2 | 130 | 300 | 18 | 2.0-7.5 | - |
JUPITER - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Jupiter C4 | 5,10,15 | 300 | 170 | 5.0 | 1.5-10.0 | L26 |
| Jupiter C5 | 5,10,15 | 300 | 170 | 5.5 | 1.5-10.0 | - |
| Jupiter C18 | 5,10,15 | 300 | 170 | 13.3 | 1.5-10.0 | L1 |
| Jupiter Proteo C12 | 4,10 | 90 | 475 | 15.0 | 1.5-10.0 | - |
GraceSmart - GRACE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| GraceSmart C18 | 3,5 | 120 | 220 | 10 | 2.0-9.0 | L1 |
Alltech® Prevail - GRACE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Prevail C18 | 3,5 | 110 | 350 | 17 | L1 | |
| Prevail C18 Select | 3,5 | 110 | 350 | 15 | L1 | |
| Prevail C8 | 3,5 | 110 | 350 | 8 | L7 | |
| Prevail Phenyl | 3,5 | 110 | 350 | 7 | L11 | |
| Prevail Cyano (CN) | 3,5 | 110 | 350 | - | L10 | |
| Prevail Amino (NH2) | 3,5 | 110 | 350 | - | L8 | |
| Prevail Silica | 3,5 | 110 | 350 | - | L3 | |
| Prevail Organic Acid | 3,5 | 110 | 350 | - | - | |
| Carbohydrate ES (polymer) | 5 | - | - | - | - |
Nano / Capillary LC Column ProteCol - SGE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| ProteCol C18 | 3 | 120/300 | 350 | 17 | 2.0-7.5 | L1 |
| ProteCol C8 | 3 | 120/300 | 350 | 10 | 2.0-7.5 | L7 |
| ProteCol C4 | 3 | 120/300 | 350 | 2.0-7.5 | L26 | |
| ProteCol SCX | 3 | 120/300 | 350 | 2.0-7.5 | L9 |
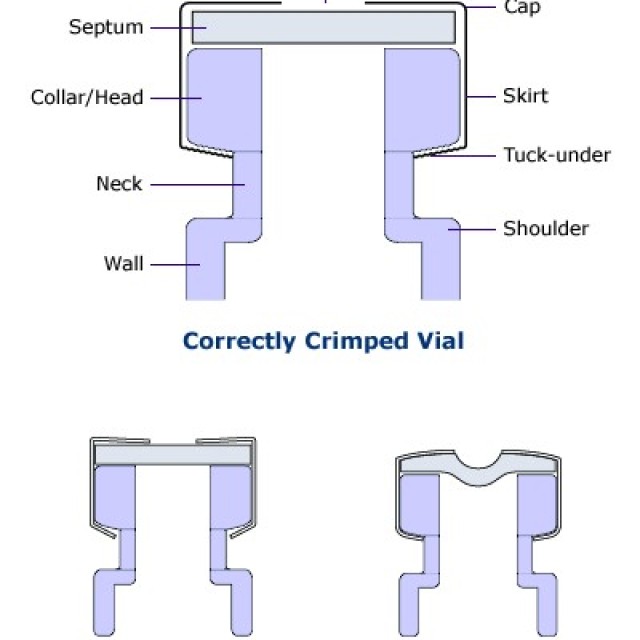
HOW TO CORRECTLY CRIMP VIALS
Crimp vials are excellent sample containers for automatic dispensers of gas and liquid chromatographs and for storing samples or calibration solutions. The technique of closing them is very important for proper tightness. Due to leakage caused by improper sealing, solvent evaporation or loss of analytes may occur.
A correctly closed vial can be recognized by the fact that its cap rotates with difficulty after closing and the septum is straight.
A vial that is closed with too much force can be recognized by the fact that its cap cannot usually be turned at all and, in addition, it has a bent septum (inwards). If the septum is punctured by the needle of the microsyringe, the septum will be heavily stressed and thus the vial's tightness will be compromised.
A vial that does not have a properly closed cap due to the low power of the crimping pliers is manifested by easy rotation of the cap and, in some cases, unfastened aluminum material around the lower edge of the vial neck.
You can set the correct force of the closing pliers.
In older types of pliers, the force is adjusted by turning the Allen key inside the jaws. Pliers also have a stop screw, which is used to set the safety distance, in order not to use too much force and thus to avoid leakage or even mechanical damage to the vial.
Mass chromatogram figures
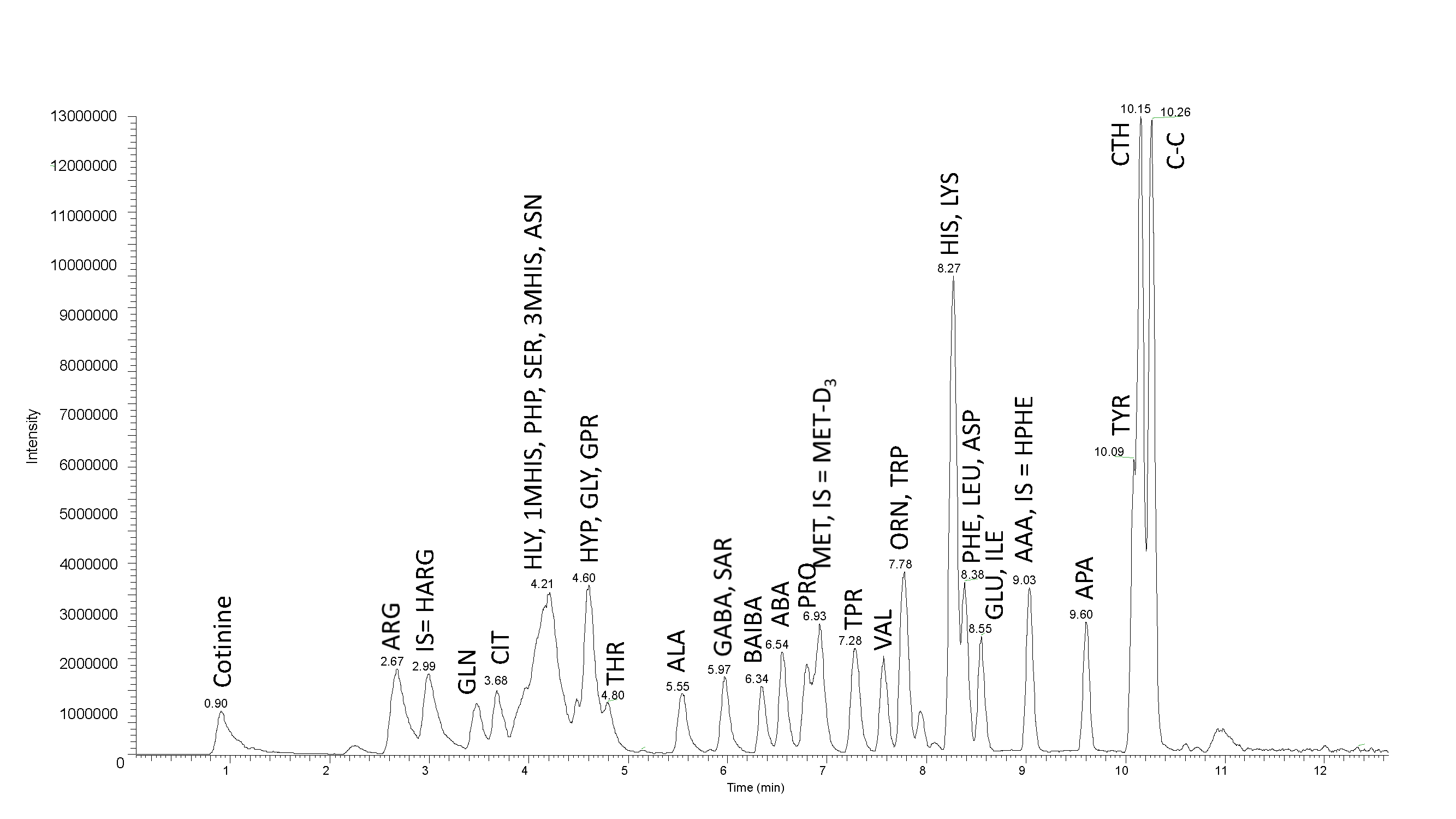
Fig. 1: Mass chromatogram of internal standards, SD1 and SD2. Separation of 5 nmoles of amino acids, using LC-MS MetAmino®
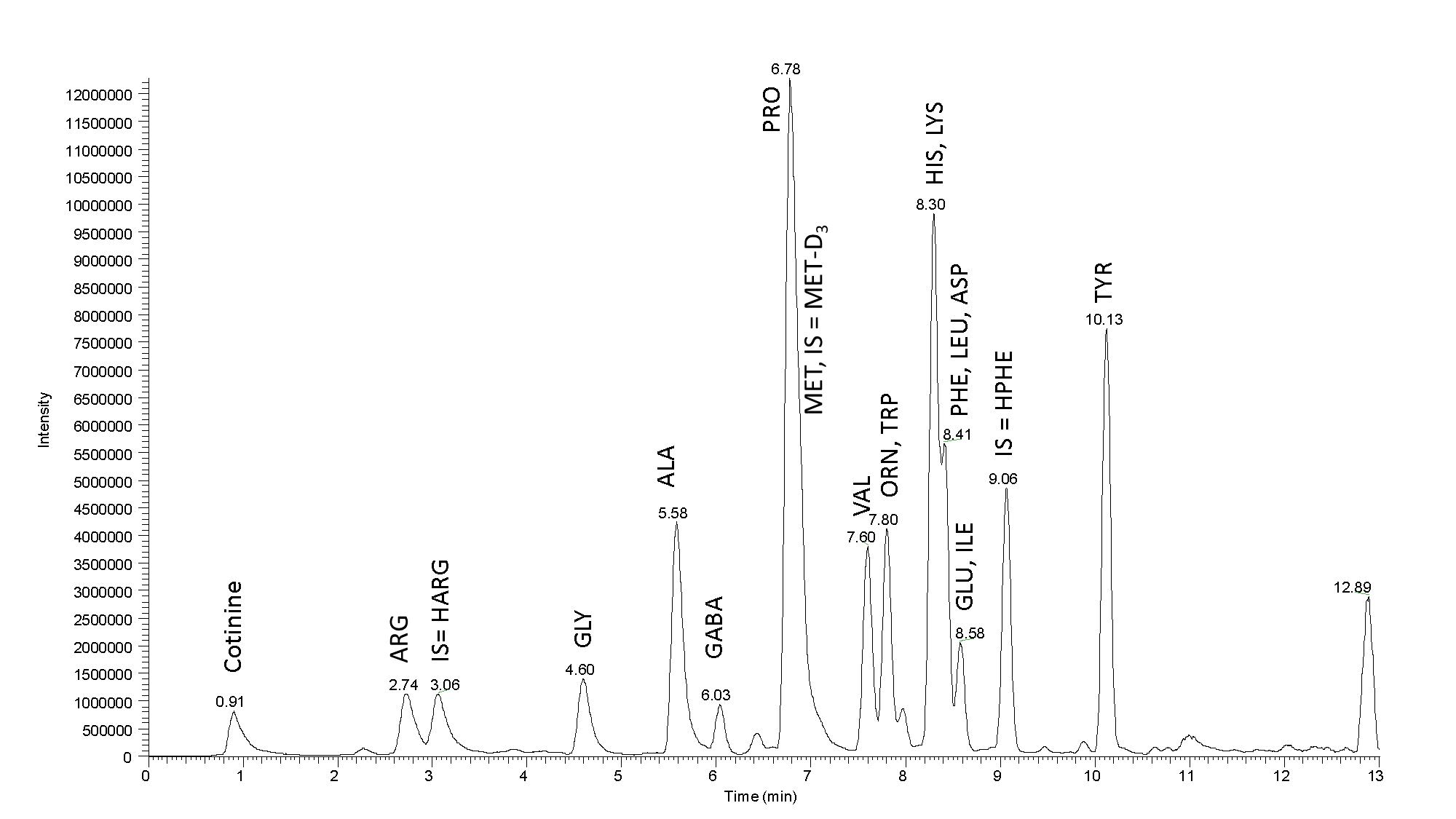
Fig. 2: Mass chromatogram a real sample: Separation of the amino acids in a sample of Budvar beer 12˚ (25 µL of the sample was the initial load). High efficiency of our column and high resolution contribute to a nice peak separation. Sample tested using LC-MS MetAmino®
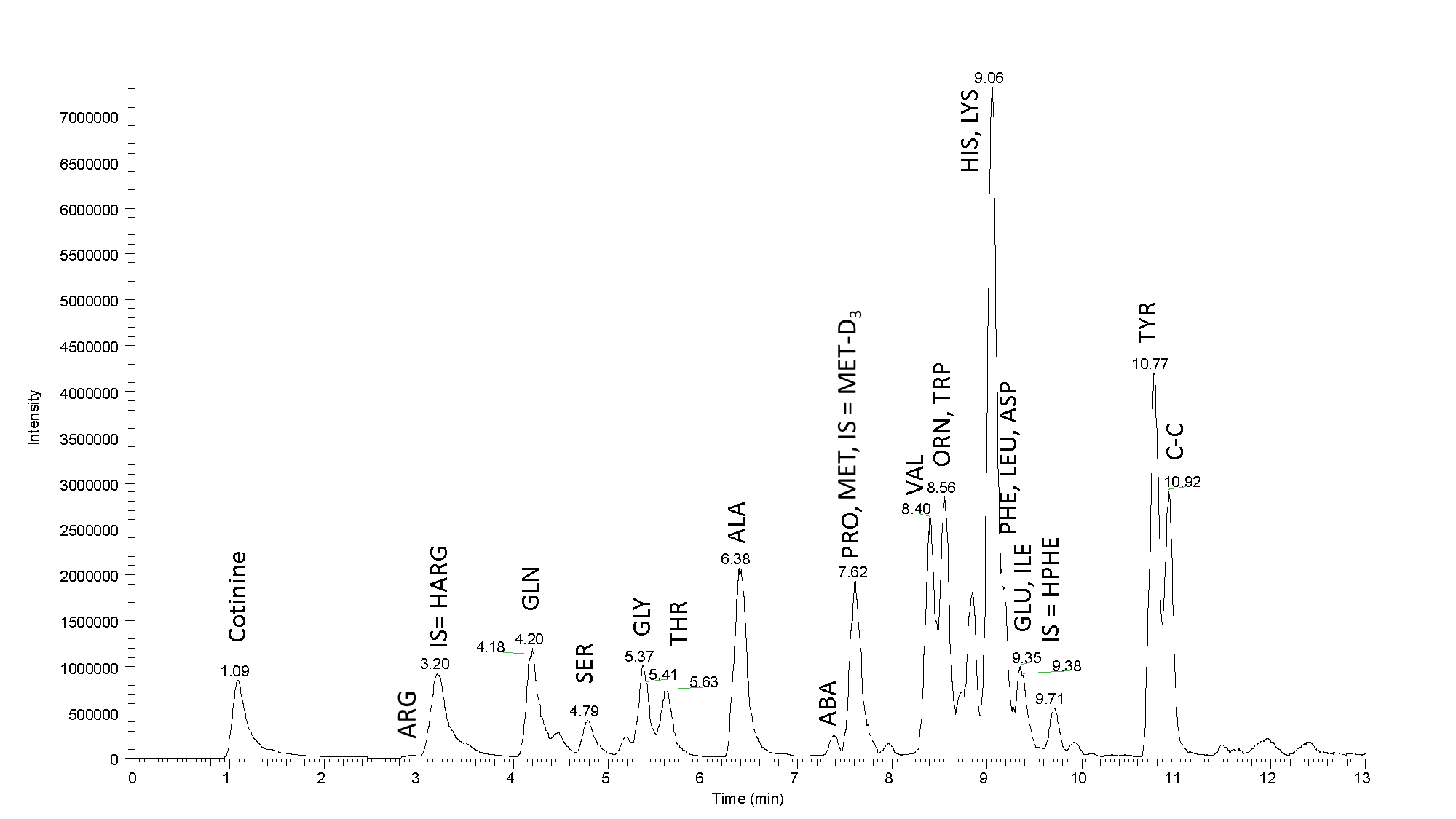
Fig. 3: Mass chromatogram of a real sample: Separation of the amino acids present in the blood serum (25 µL of precipitated serum was the initial load). High efficiency of our column and high resolution contribute to a nice peak separation. Sample tested using LC-MS MetAmino®
Sample preparation
After extracting the content out of your matrix, the sample preparation for LC-MS/GC-MS analysis can take place.
This protocol only briefly describes the sample preparation procedure. For detailed procedure see the Metamino® user manual.
LC-MS analysis goes as followed:
- Pipette 100 μL of your extracted content (serum, plasma, ...) and add 100 μL of precipitation medium. Centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Pipette 25 μL of your precipitated sample and add 10 μL of solution with internal standard to each sample preparation vial.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5-10 sec.
- Pipette 10 μL of reagent solution into each sample preparation vial and vortex 5-10 sec. Let the derivatization proceed for at least 2-3 minutes.
- Activate and equilibrate the sorbent in microspin filter by adding:
- 200 μL of MSPE activation medium, then centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- 200 μL of MSPE sorbent equilibration medium, then centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Discard the flow through.
- Dilute derivatization reaction mixture with 400 μL of diluting and washing medium and vortex 5-10 sec.
- Load the diluted reaction mixture (typically 450-500 μL) to the wetted microspin filter sorbent and let it stand for 1-2 min. Centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Discard flow through.
- Wash the sorbent in the microspin filter with 200 μL of diluting and washing medium and centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Place the microspin filter into a new centrifugal vial, add 200 μL of eluting medium and centrifuge 30 to 60 sec at 1,500 ×g (6,000 rpm).
- Transfer the eluate into the autosampler vial. The sample is ready for LC-MS analysis.
GC-MS analysis goes as followed:
- Pipette 25 μL of your target sample and 10 μL of solution with internal standards into each reaction glass tube.
- Pipette 20 μL of reducing agent into each reaction glass tube and vortex briefly 5 to 10 sec. Let it stand for 1 to 2 minutes.
- Pipette 25 μL of basic medium into each reaction glass tube and add 50 μL of reagent (derivatization) solution into each reaction glass tube and vortex 5 to 10 sec.
- Pipette 50 μL of reagent (derivatization) solution medium into each reaction glass tube and vortex 5 to 10 sec.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5 to 10 sec.
- Pipette 25 μL of catalytic solution into each sample preparation vial and vortex 5 to 10 sec. Let it stand for 1-2 minutes. The emulsion will gradually separate into two layers.
- Pipette 50 μL of extraction medium into each sample preparation vial and vortex 5 to 10 sec.
- Pipette 25 μL of acidic medium into each sample preparation vial and vortex 5 to 10 sec. Then centrifuge 30 to 60 sec 1,500 ×g (6,000 rpm).
- Transfer the organic (upper) layer (50-100 µL) into the autosampler vial with insert. The sample is ready for GC-MS analysis.
Kit content
The MetAmino® kit includes all reagents, liquid media and chemicals. Start-up kit content is listed in tables below:
LC-MS kit content for 100 samples:
| Item | Vial type | Volume in vial (mL) | No. of vials (100 samples) |
| Amino acid standards SD1 solution | 2 ml vial | 0.25 | 1 |
| Amino acid standards SD2 dried | 2 ml vial | - | 2 |
| Solution with internal standards | 2 ml vial | 1.1 | 1 |
| Amino acid standard diluting medium | 4 ml vial | 1.4 | 1 |
| MSPE sorbent activation medium (WES) | 40 ml vial | 22 | 1 |
| MSPE sorbent equilibration medium (EQS) | 40 ml vial | 22 | 1 |
| Catalytic Solution (CTS) | 4 ml vial | 2.2 | 1 |
| Reagent (Derivatization) Solution (RDS) | 4 ml vial | 1.1 | 1 |
| Diluting and Washing Medium (DWM) | 40 ml vial | 33 | 2 |
| Eluting Medium (ELM) | 40 ml vial | 22 | 1 |
| Precipitating Medium (PM) | 40 ml vial | 11 | 1 |
| Item | Amount (100 samples) | Note |
| MetAmino® LC Column | 1 pc | Proprietary stationary phase |
| Reagent tray for up to 80 Centrifugal Tubes | 1 pc | See Fig. 5 in Section 5.1 |
| Microspin Filters with the MetAmino® sorbent | 100 pcs | Inner tube incl. 0.22 µm membrane |
| Centrifugal Tubes (2 mL) | 400 pcs | Outer tube |
| Autosampler Vials (9 mm screw-top) | 100 pcs | Including septa and caps |
GC-MS kit content for 100 samples:
| Item | Vial type | Volume in vial (mL) | No. of vials (100 samples) |
| Amino Acid Standards SD1 Solution | 2 ml vial | 0.25 | 1 |
| Amino Acid Standards SD2 Dried | 2 ml vial | - | 2 |
| Solution with Internal Standard (IS) | 2 ml vial | 1.1 | 1 |
| Amino Acid Standard Diluting Medium (AASDM) | 4 ml vial | 1.4 | 1 |
| Reducing Agent (RA) | 4 ml vial | 2.75 | 1 |
| Basic Medium (BM) | 4 ml vial | 2.75 | 1 |
| Catalytic Solution (CTS) | 40 ml vial | 5.5 | 1 |
| Reagent (Derivatization) Solution (RDS) | 40 ml vial | 5.5 | 1 |
| Extraction Medium (EM) | 40 ml vial | 5.5 | 1 |
| Acidic Medium (AM) | 4 ml vial | 2.75 | 1 |
| Item | Amount | Note |
| MetAmino® GC Column | 1 pc | Proprietary stationary phase |
| Reaction glass tubes | 100 pcs | - |
| Reagent tray for up to 80 reaction Tubes | 1 pc | See Fig. 3 in Section 5.1 |
| Autosampler Vials (9 mm screw-top) | 100 pcs | Including septa and caps |
| Inserts for Autosampler Vials | 100 pcs | - |
Description
Our LC-MS/GC-MS MetAmino® kits provide fast, robust, reproducible and accurate procedure for the amino acid quantification, where both sample handling and chromatographic separation are taken into account.
Using liquid chromatography (LC) coupled to mass spectroscopy (MS), the method is based on micro-solid phase extraction (MSPE) using special MetAmino® spin filter with proprietary media. MetAmino® microspin filter absorbs derivatized amino acid analytes, which are eluted through the integrated 0.22 μm membrane and afterwards injected into a MetAmino® HPLC column, undergoing a LC-MS analysis. In total, sample preparation takes around 8 minutes and sample analysis 12 minutes, thus the entire experimental time is only 20 minutes.
While using gas chromatography (GC) coupled to MS, the sample is firstly derivatized followed by liquid-liquid microextraction step (LLME). Derivatized analytes migrate into the organic layer which is then directly amenable for a quick detection on GC-MS system. In GC-MS kit, sample preparation time has been shortened to 5 minutes and sample analysis runs 12 minutes. Total experimental time is roughly 17 minutes.
This unique kit meets all emergency requirements and needs of high throughput laboratories and is designed for all end-users seeking high separation efficiency.
Overview of MSPE sorbents
Sorbents for the MSPE technique are chosen to cover the widest possible field of applications. MSPE SpeExtra C18 is a hydrophobic type of octadecyl silica gel with a special endcapping. It is suitable for a wide range of analytes, showing lower retention for polar compounds. MSPE SpeExtra C18-P is a polar modified monomeric octadecyl silica gel. It offers different types of interactions: dipole-dipole, π-π and hydrophobic. It is therefore suitable for aromatic and polar compounds. MSPE SpeExtra HLB polymer sorbent with high specific surface area and special endcapping. It has a hydrophilic and lipophilic modification ensuring universal use and a higher capacity than C18 silica gel. MSPE SpeExtra WAX is a PS/DVB-based polymer sorbent with special end-capping and a highly specific surface, which is prepared by copolymerization of styrene and divinylbenzene, creating weak anionic interactions.
| MSPE sorbent | Particle size [µm] | Specific surface area [m 2 /g] |
| C18 | 60 | 310 |
| C18-P | 60 | 310 |
| HLB | 30 | 850 |
| WAX | 30 | 850 |
Purification & testing for cannabis and hemp
 Hemp contains hundreds of cannabinoids with Cannabidiol (CBD) being the most prevalent in the plant and Δ9-Tetrahydrocannabinol (THC) being the active ingredient causing psychotropic effects. However, many more compounds are formed by the hemp plant and have been investigated for their medical effects. This limit often requires THC remediation of the distilled hemp extract (starting material) and can be achieved using preparative scale chromatography such as the puriFlashR XL-Cannabis system. HPLC analysis of the starting material (third pass distillate), fractions collected during the remediation process, and the finished product can be performed using the Advion AVANT HPLC-UV analytical system. Both the purification and analytical processes are shown in this application note to form a complete solution for THC remediation in the hemp industry.
Hemp contains hundreds of cannabinoids with Cannabidiol (CBD) being the most prevalent in the plant and Δ9-Tetrahydrocannabinol (THC) being the active ingredient causing psychotropic effects. However, many more compounds are formed by the hemp plant and have been investigated for their medical effects. This limit often requires THC remediation of the distilled hemp extract (starting material) and can be achieved using preparative scale chromatography such as the puriFlashR XL-Cannabis system. HPLC analysis of the starting material (third pass distillate), fractions collected during the remediation process, and the finished product can be performed using the Advion AVANT HPLC-UV analytical system. Both the purification and analytical processes are shown in this application note to form a complete solution for THC remediation in the hemp industry.
- puriFlah® L-Canabis instrument with the output up to 4.3 kg/day (columns up to 15 cm ID)
- puriFlah® XL-canabis instrument with the output up to 12.2 kg/day (columns up to 20 cm ID)
- Plate Express TLC Plate Reader
- Show all

Sensogate WA 130 fully automated fitting for 12mm OD sensor with PG13,5, SS 1.4571, Ingold socket
Availability: ask uson inquiry





 0
0
 0
0