
Process measurement of liquids
Automatic cleaning and calibration of pH sensors in process measurement
In process pH measurement, we often encounter a problem where relatively frequent pH sensor maintenance is required. Unfortunately, when handling the sensor, it happens to be damaged. In order to avoid it and to make more frequent maintenance without human intervention, it is possible to invest in technology to create a system that will ensure:
- Automatic measurement
- Automatic sensor cleaning without human intervention
- Automatic calibration
- Replace sensor without shutting down

Such a system can be built with Knick's innovative solution. This solution is based on a set consisting of:
- Intelligent Protos converter
- Automatic pull-out fitting Ceramat or Sensogate
- Unical 9000 system
- Sensor Memosens
The system can be installed directly in operation, both in a safe environment and in a potentially explosive atmosphere. The fittings can be placed both directly into the pipeline and into the reactor. The connections can be different, starting with the standard flange with Variline special connections.
CHS UVSens photometer for COD measurement
 CHS UVSens is a small and light photometer designed for measuring organic water pollution. Measurement of water quality parameters takes place on the basis of absorbed light with a wavelength of 254 nm .
CHS UVSens is a small and light photometer designed for measuring organic water pollution. Measurement of water quality parameters takes place on the basis of absorbed light with a wavelength of 254 nm .
- A complex mixture of organic components, commonly found in water, causes problems in water management
- Many of these substances absorb UV radiation of 254 nm
- We can determine these substances by means of radiation absorption
- CHS UVSens – fast and safe optical measurement
Important concepts
| UVT | Amount of light passed through the sample = transitance |
| UVA | Amount of light absorbed = absorbance [2-log 10 (UVT)] |
| SAC 254 | Spectral absorption coefficient (UV 254) |
| SUV | Specific UV absorbance (UVA/DOC) |
The photometer allows two-point calibration. Calibration, or the conversion of the UV 254 nm absorbance value to the required total parameter depends on the composition/origin of the water.
- Different locations require their own calibrations
- Some locations may need multiple calibrations depending on changing conditions (e.g. season)
CABLES AND CONNECTORS FOR CHS SENSORS
Continuous measurement
Probes with a connector (S8, VP6, Memosens,…) and a cable fixed in the terminal board of the converter are standard.
S8*
- two-pole basic connector
- can transmit only one signal, so it is suitable where a temperature sensor is not needed or it is located outside the pH probe
- for humid environment can be used with a suitable armature, for example CHS WaterDip
- cable:
- diameter 3 mm, weak shielding - prone to electromagnetic interference
- recommended length up to 5 meters
- installation: away from sources of interference (power cables, electric motors, transformers)
- prone to stray currents on metal constructions
VP6
- VarioPin connector with 6 pins can transmit multiple signals. Suitable for probes with integrated temperature sensor
- robust metal construction and good water resistance
- use in almost all environments and applications
- cable:
- double shielding, less prone to electromagnetic interference
- recommended length up to 20 meters
installation: away from sources of interference (power cables, electric motors, transformers)
Memosens
- inductive connector
- signal transmission does not provide by contact of metal components, but by induction between the coils in the probe and the cable
- resistant to almost any environment, it does not mind moisture or corrosive substances
- the digital signal is resistant to any interference
- suitable for any application, especially in humid and corrosive environments
- cable:
- the digital signal is not prone to interference
- recommended length up to 20 meters
- installation: also possible in cable ways with power conductors
Probes with a fixed cable are used rather seldom in case of simpler measuring systems. Usually for little sophisticated converters, where the signal cable is connected to an external (usually BNC) connector. This is a source of problems due to environmental corrosion of the connector.
Picture of sensor´s connectors:
 |  |  |
| connector S8 | connector VP6 | connector Memosens |
Laboratory and portable measurements
Probe with connector (usually S7*)
- used as an economical variant where an external temperature sensor is placed or temperature measurement is not required
- two-pin S7 connector can only transmit one signal
- recommended as laboratory solution
- in case of interference is recommended to use a braided cable (5 mm)
Fixed cable
- is required in case of integrated temperature sensor
- suitable for portable measurements
- the absence of a connector is good for humid environments
- if the entire probe is accidentally immersed in the sample, a risk of leakage is not as high as in case of connector
- there is no risk of probe´s breakage when connector is released
Connectors on the instrument side which are usually used for pH measurement:
| Connector | Description |
| BNC | bayonet two-pole connector: central pin (measuring electrode signal) and bayonet (reference signal - shielding) |
| DIN | coaxial antenna connector: central pin (measuring electrode signal) and ring (reference signal - shielding) |
Connectors such as Cinch, Jack, "banana" and others are commonly used to transmit the temperature sensor signal. Usually two-pole versions, or two separate bananas. In the case of only one pole, the (-) pole of the temperature measurement is common to the reference of the pH probe. It depends on the manufacturer of the pH meter.
Note:
* S7/S8 cable part of connector is identical, the difference is in the head of probe
– S7: laboratory version
– S8: process type with thread (PG 13.5) for pipe mounting.
 |  |
| connector BNC | connector DIN |
pH measurement
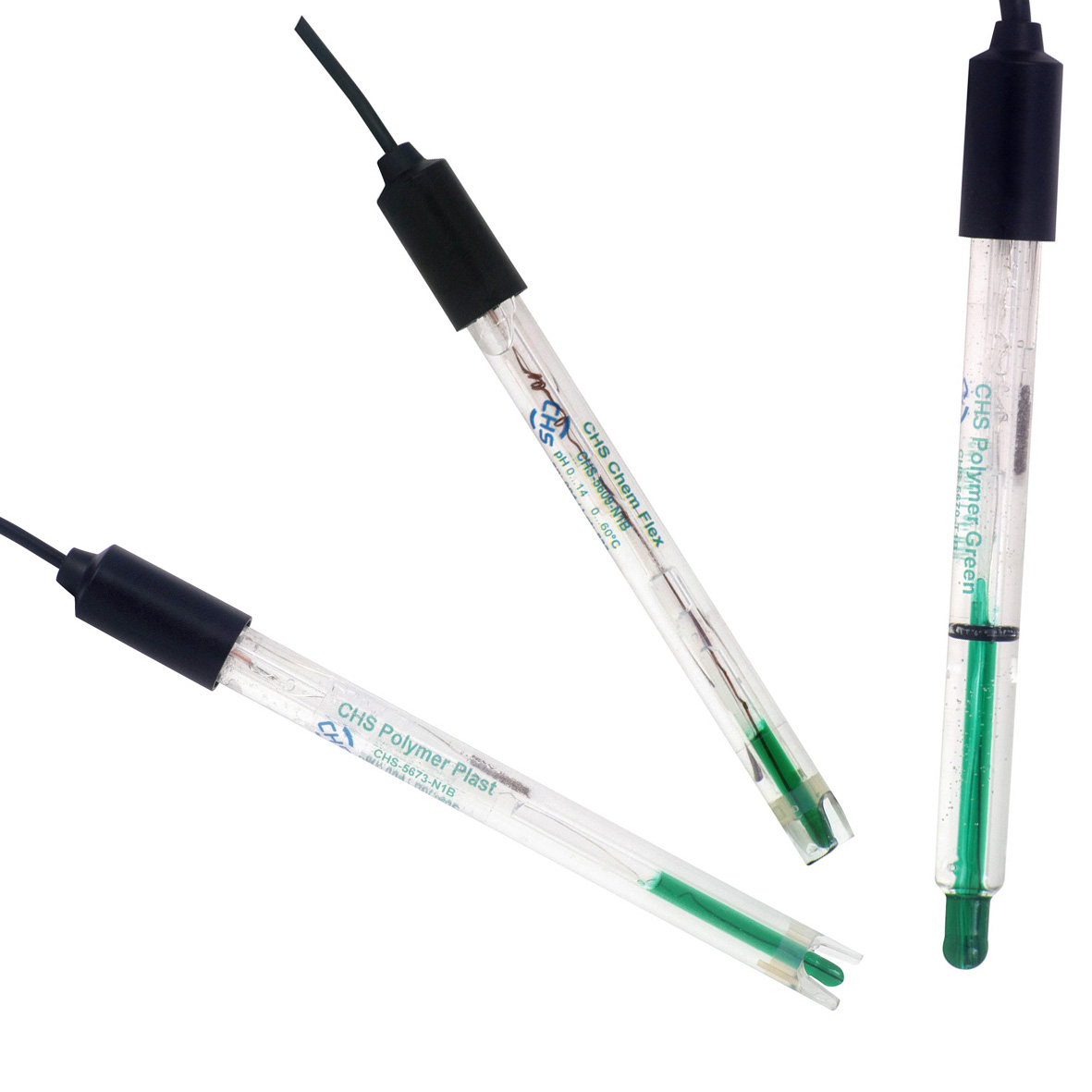 The pH Measurement
The pH Measurement
The determination of the pH values is based on the principle of the potentiometric measurement - the measurement of electrical voltage. A pH electrode consists of two electrodes (pH glass membrane and reference) that are combined into one device in a combination pH electrode. Between these two electrodes a voltage is measured. The pH membrane of the electrode is made of special glass that is impermeable and electrically isolated. This glass (pH glass) forms a hydrated layer in water and responds selectively to hydrogen ions (H+). Sodium ions (Na+) of the glass are replaced by hydrogen ions (H+), which causes a change in free energy and an electrical potential that the pH meter measures. The amount of Na+ and H+ exchange across the pH glass depends strongly on the pH of the solution. The higher the pH the less hydrogen ions are in the solution, therefore less sodium ions are replaced across the pH glass. The liquid inside the pH glass is a buffer solution with a known and constant hydrogen ion concentration. Depending on the difference in pH between the inner buffer and the measuring solution a galvanic voltage is going to be produced between the inner and the outer layer of the pH glass. This voltage is measured by two Ag/AgCl electrodes. one electrode is located in the inner buffer the other in the reference electrolyte. Most pH electrodes have a nearly linear behavior in the measuring range of pH 0 to 14. Taking advantage of this behavior a pH electrode is calibrated between two different well known pH values, for example pH 4.01 and 7.00. Between those two values a linear extrapolation and also a linear interpolation is conducted.
Dissolved oxygen measurement
Optical sensors for measuring dissolved oxygen
Previously used amperometric probes use current measurement between two electrodes immersed in an electrolyte solution using a so-called Clark measuring cell. A typical cell consists of a gold cathode and a silver anode to which a bias voltage (approximately 0.8 V) is applied. The electrodes are immersed in an electrolyte based on an aqueous solution of KCl or KBr and are separated from the measured medium by a semi-permeable polymer membrane that lets in oxygen. Oxygen dissolved in the liquid medium diffuses through the membrane into the electrolyte, and as a result of oxidation-reduction reactions on the electrodes, a very small current (size of tens of nanoamperes) passes through it, directly proportional to the oxygen content in the electrolyte, and therefore the concentration of dissolved oxygen in the measured medium.
Principle of measurement
Optical sensors for measuring dissolved oxygen work on a completely different principle than previously used amperometric probes. Some manufacturers use two different light-emitting diodes (LEDs). For the CHS ODOSens product, our company uses double detection of luminescent radiation (excitation LED and detection diodes), which minimizes the effect of sample inhomogeneity and possible damage to the luminophore. The integrated converter constantly compares the outputs from both measuring elements aimed at other parts of the luminophore and thus ensures the most accurate measurement of the dissolved oxygen concentration.
Sensors of the CHS ODOSens series measure, similarly to sensors with Clark's whole, the partial pressure of oxygen, which can be expressed as a percentage of air saturation or as a concentration in units of micrograms per liter (ppb, i.e. 1x10 -9 ), or mg/l (ppm, i.e. 1x10 -6 ). It can be measured in the range of concentrations from 8 ppb to 25 ppm, which corresponds to the range from 0.1% to 300% air saturation. For most applications, this measuring range is more than sufficient, but the CHS ODOSens T sensor, which reliably measures even 1 ppb, is offered for special applications (e.g. brewing).
Literature:
KADLEC, K.: New optical sensors of the amount of dissolved oxygen. Automa, 2007, No. 12, p. 46 (the article cannot be found in the publisher 's archive)
Can you trust your buffer solution?
GMP, GLP, ISO 9001, EN 45000, Calibration, Verification, Traceability, and Certification from an accredited organization: Key words those are increasingly important. The calibration of pH and Redox electrodes has never been easy. All calibration procedures assume that the labelled values of the calibration buffers are correct. But buffer values can change over time and so can your results
A complete range of patented buffer solutions provides never before achieved pH stability. Hamilton guarantees DURACAL pH buffers for 5 years after the date of manufacture. The pH 9.21 and pH 10.01 buffers are even stable in air. See the diagram below for details. High buffering capacity provides rapid, stable calibration. Preservatives are added to prevent microbial and mold growth.
Traceability
An important issue for the production of Certified Reference Material is to ensure the traceability through an unbroken chain of comparisons to reference material of the highest metrological quality (Primary Reference Material).
- Closed Loop Traceability: Unlike other manufacturers where only a top-down traceability is applied, Hamilton is working with circular or closed loop traceability. The closed loop traceability ensures the users of Hamilton DURACAL buffer a unique reliability!
- Top-down traceability: At Hamilton, the pH value of DURACAL buffers is determined by comparison against two Secondary Reference Buffer Solutions. These are purchased from accredited suppliers for Secondary Reference Materials. The solutions themselves are compared against Primary Reference Solutions from PTB1) or NIST 2).
- Bottom-up traceability: To ensure the highest possible accuracy and full reliability of the pH value, a representative number of samples from every single production lot is sent to a German DKD3) laboratory (DKD-K-06901) for an external, independent and impartial verification. In this laboratory, the DURACAL samples are compared against Secondary Reference Solutions from DKD-K-06901.
The Secondary Reference Solutions are of course compared against Primary Reference Solutions from PTB. At this stage, the loop is closed: the PTB Primary Reference Solution is the starting and ending point of the traceability loop. DKD provides Hamilton with a calibration certificate for every DURACAL production lot.
Cleaning & reconditioning pH electrodes
 Often the pH meters are used in applications, which require regular cleaning of the electrode. These applications involve very hard waters, dirty samples like soil slurries, viscous materials or samples with high oil and protein content. We do not recommend these procedures for persons unfamiliar with or unable to use safe techniques involving these chemicals: Detergents, HCl (Hydrochloric Acid), and NaOH (Sodium Hydroxide).
Often the pH meters are used in applications, which require regular cleaning of the electrode. These applications involve very hard waters, dirty samples like soil slurries, viscous materials or samples with high oil and protein content. We do not recommend these procedures for persons unfamiliar with or unable to use safe techniques involving these chemicals: Detergents, HCl (Hydrochloric Acid), and NaOH (Sodium Hydroxide).
 Method 1
Method 1
Soak the electrode in a 0.4 molar concentration of HCl (hydrochloric acid) for 10 minutes, then rinse the electrode with deionized or distilled water. This should remove any organic protein from the glass electrode and the surface of the reference electrode.
Method 2
Soak the electrode in a 3.8 or 4.0 molar KCl (potassium chloride) solution heated to 50°C for 1 hour. Allow the KCl solution to cool down to room temperature, then rinse the electrode with deionized or distilled water. This will open and clean the reference electrode of all contaminants.
Method 3
Soak the electrode in a 4.01 pH buffer solution (EC-BU-4BT), heated to 50°C for 1 hour. Allow the buffer to cool down to room temperature, then rinse the electrode with deionized or distilled water. This will open and clean the reference electrode.
Method 4
After each use, rinse the electrode in 0.5 N or 1% HCl. If you have a build-up of oil or protein contaminants, try soaking the electrode in warm detergent and water solution. Degreasing dishwashing detergents or stain removing pre wash pretreatment are ideal for this: any brand will do. An overnight soak may be needed if build-up is heavy. Then rinse the pH sensor in deionized or distilled water and soak for 10 minutes in 1% HCl. Rinse the pH sensor in deionized or distilled water and check in buffers. If the pH sensor calibrates to buffers it can be used in tests. When the pH electrode cannot be calibrated even after attempts to clean it, it must be replaced.
Method 5
For protein removal, soak the pH electrode in contact lens enzymatic cleaner solution overnight. The enzymes will remove proteins from glass and plastic.
pH electrode maintenance
 Maintenance and storage of pH electrodes
Maintenance and storage of pH electrodes
pH electrodes are delicate sensors that require proper care and maintenance to produce accurate and reliable results, and to prolong useful life. When the pH electrode is not used for a period of time, always keep it moist. Store the pH electrode in storage solution or in a pH 7 buffer. DO NOT store the electrode in distilled or deionised water as this will cause ions to leach out of the glass bulb and reference eledtrolyte, causing slow and sluggish response.
pH electrodes may be shipped with either protective caps or in electrode soaking bottles to prevent cracking or scratching, and to keep the glass bulbs moist. Remove the electrode gently from the storage bottle and rinsse it with distilled water before use. For long-term storage, always keep the pH electrode in the bottle, filled with sufficient storage solution to cover the glass bulb. Replenish the bottle as needed.





 0
0
 0
0


